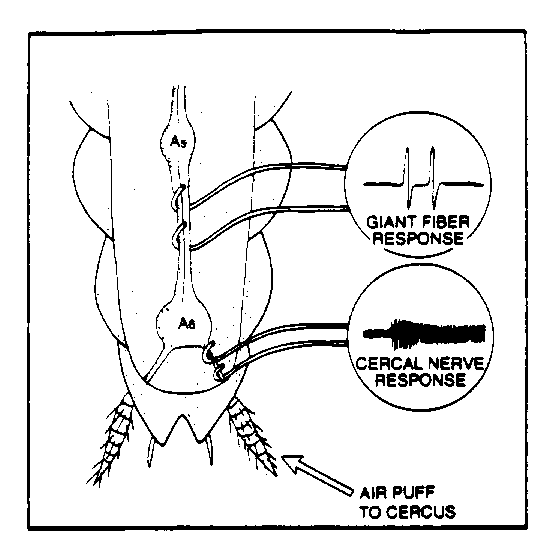
Fig. 1. Electrode placement for ventral and cercal (week 2) nerve recording.
Lab #4
Cockroach Ventral Nerve II
Equipment Needed Per Rig:
Standard. MacLab data acquisition system and Macintosh, MacLab Chart and Spike histogram extension software, 1 DAM60 Amp and connectors (one additional DAM60 needed for the lab for the conduction velocity portion of the protocol), oscilloscope and audio monitor, BNC cables, barrels, and Ts.(see Equipment page for model information etc. for lab report), male banana to BNC adapter
Special. 1 minuten pin electrode (for ground), 1 silver-silver chloride hook electrode (one additional hook electrode per lab for the conduction velocity portion), dental wax, cockroach perfusion solution (CPS), air flow meter, PE-205 tubing, silver silver-chloriding solution (0.2 M NaCl), cigarette or cigar for blowing nicotine onto the terminal ganglion, 2% flaxedil, TTX (2 micromolar) solution.
Introduction
In last week's lab we learned how to prepare a cockroach for ventral nerve recording, record from the ventral nerve, and stimulate the cerci to measure activity of the nerve. In this lab we do the same dissection, but now go a bit posterior to expose the very fine cercal nerves. The cercal nerve is a bit more superficial and since it is so thin this is a challenging dissection. After recording from a cercal nerve, using puffs of air to observe the activity, we will move over to the ventral nerve and compare the recordings from the two different nerves. Then we will start applying various neuroactive solutions.
As with last week's lab, keep these things in
mind as you follow the protocol below:
1) Be very conservative in the amount of CPS you apply to the animal: if you get the cerci wet it will be very hard to get good responses to wind puffs.
2) Lack of response from the nerve cord is often attributable to the electrodes
shorting out from touching each other or being immersed in a conducting
fluid such as CPS.
3) Stabilizing the holder (petri dish) with dental wax along the base of
the dish will help eliminate accidental movement of the apparatus (usually
resulting in nerve injury).
4) Depending upon the stability of the preparation, you may need to push
air over the cerci in a direction away from the open nerve recording. This
can help eliminate undue movement of the recording leads and subsequent
baseline shifts.
5) Be very conservative in the number and amount
of wind puff stimulation, as the nerve can very quickly be rendered unresponsive
and will need a period of recovery to return to normal sensitivity.
Dissection
Prepare the animal for recording as you did last week, and expose the ventral nerve. DO NOT APPLY THE MINERAL OIL MIXTURE. We need to keep the body cavity clean for the various solutions Then, very carefully expose the fine cercal nerves that are just caudal to the terminal ganglion, as shown in Fig. 1. below. Keep in mind the nerve is a bit more dorsal than the ventral nerve.

Fig. 1. Electrode placement for ventral and cercal (week 2) nerve recording.
Experimental Protocol:
1) Cercal nerve and ventral nerve air puff response. Make sure that the cerci are completely free of liquid and not touching anything (dish, pins, etc.). You may need to use a sipper/pippette to remove excess fluid. Using the supplied PE-205 tubing, blow on the cerci and observe the response of the cercal nerves. Apply 3 to 5 puffs and digitally acquire the resulting response. Since we are recording one channel of data, you can go into to "fast" acquire mode and accumulate 16,000 samples at high resolution (try 10K samples/sec). You'll have to time your (brief!) puff accordingly if you do this. Now, move your hook electrode to the ventral nerve and again acquire approximately 5 puff responses. In your lab report, discuss the differences in the observed cercal and ventral nerve responses to the air puffs. Discuss what the transfer function of the abdominal ganglion may be, based on your recording. What is the neural and behavioral significance of this transfer function?
2) Response of ventral nerve to application of neuroactive solutions. We will begin with excitatory stimulation of the terminal abdominal ganglion.
Since the ganglion is sheathed, we will try one or both of the following methods of getting our solutions perfused into the ganglion: a) cutting of peripheral nerves coming from the abdominal ganglion (but NOT the cercal nerves) in order to provide portals into the ganglion or b) using a minuten pin, scratch the sheath gently, this will tear it and allow perfusion to occur. Attempt these interventions in the order presented.
2 .1 Using the supplied PE tubing, blow several puffs of cigarette or cigar smoke onto the ganglion while recording the spontaneous activity (continuous mode: close filters and increase gain to stretch the action potentials to fit in the 1K sample slices). Alternatively, or in addition, apply a few microliters of .1% ethanol. How does the spontaneous rate change? How does the response to brief light puffs of air to the cerci change? Quantitate this using the ratemeter or ISIH functions of the Spike Histogram Extension. Explain the observed changes, if any. Now try to reverse the effect by washing out the substances with CPS and absorbing the excess with twisted Kimwipes.
2.2 (GROUP A) Once your nerve has returned to pre-stimulation spontaneous activity, apply a few microliters of curare (2% flaxedil solution) to the ganglion. Record the pre- and post-application spontaneous activity. Applying brief puffs of air, observe whether the response changes compared to untreated response. Again, quantitate and explain the change in baseline rate, if any.
2.2 (GROUP B) Once your nerve has returned to pre-stimulation spontaneous activity, apply a few microliters of TTX (2 micromolar) to the ganglion. Applying brief puffs of air, observe whether the response changes compared to untreated response. Record the pre- and post-application spontaneous activity. Again, quantitate and explain the change in baseline rate, if any.
LABORATORY REPORT
Your lab report will be due the lab after the next lab meeting, and will
cover both cockroach lab days.
Follow the standard journal format described in the syllabus.
EXTRA CREDIT QUESTION
Every organism has a rapid response system to threatening stimuli. The responses generally fall into one of fight, flight, or freeze. One would expect there to be fairly tight coupling between the behavioral ecology of an animal and the sensory receptors and accessory organs that mediate the animals' escape behavior, since failure to escape usually means death. Rabbits, for example, have given up forward looking eyes with high acuity (what visually guided predators often have) in favor of eyes positioned on the sides of their head with high sensitivity (and thus low acuity), as befits an animal that often has to escape from a variety of visually-guided predators.
What do the characteristics of the sensory receptors and structures that mediate cockroach escape behavior tell you about the behavioral ecology of these arthropods?
WWW resource. A useful web page on the escape response of the American Cockroach.