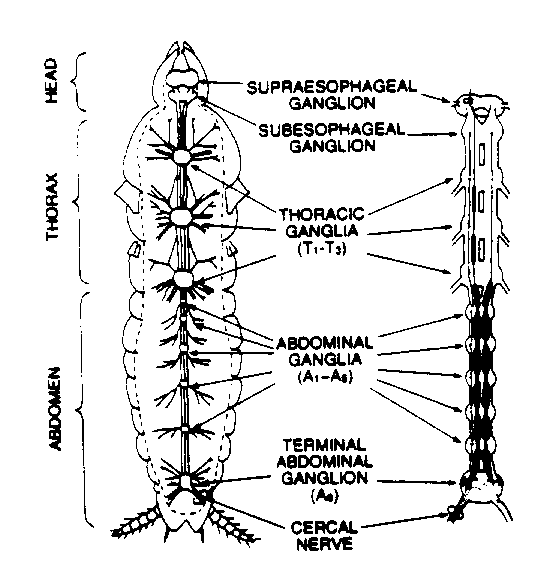
Fig. 1: Central nervous system of a cockroach
Lab #3
Cockroach Ventral Nerve I
Equipment Needed Per Rig:
Standard. MacLab data acquisition system and Macintosh, MacLab Chart and Spike histogram extension software, 1 DAM60 Amp and connectors (one additional DAM60 needed for the lab for the conduction velocity portion of the protocol), oscilloscope and audio monitor, BNC cables, barrels, and Ts.(see Equipment page for model information etc. for lab report), male banana to BNC adapter
Special. 1 minuten pin electrode (for ground), 1 silver-silver chloride hook electrode (one additional hook electrode per lab for the conduction velocity portion), dental wax, cockroach perfusion solution (CPS), Vaseline-mineral oil mixture in a syringe, flow meter, PE-205 tubing, silver silver-chloriding solution (0.2 M NaCl).
Introduction
The cockroach ventral nerve cord is a complex neuronal system. However, one example of neuronal integration can easily be explored with this preparation; the conversion of mechanosensory input from primary sensory fibers into responses of giant interneurons. The mechanosensory input is derived from the stimulation of the pair of appendages at the tip of the abdomen called the cerci. Each cercus bears a large number of long tactile hairs which are so sensitive they may be stimulated by not only direct touch, but also by vibrations of the substrate upon which the insect rests, or by air movements, including low frequency sound waves. Through neuronal processing thought to be at the terminal ganglia, the cerci work in concert to determine the velocity and direction of the stimulus disturbing the hair cells (usually air displacement caused by an oncoming predator). The cerci have on their surface hundreds of tiny hairs, each connected to individual mechanoreceptors. The axons of the mechanoreceptors project to the sixth (or terminal) abdominal ganglion where they make synaptic connections with two sets of giant nerve fibers (Figure 1). The giant fibers then project to the thoracic ganglia and the head and are involved in the coordinated evasive response elicited by cercal stimulation. The focus of this laboratory experiment is to correlate the escape response elicited by stimulation of the cerci to the electrophysiological activities of the ventral nerve cord and cercal nerves during cercal stimulation. We will also measure the conduction velocity of the ventral nerve to estimate the minimum time needed by the animal to escape. The stimulation of the cerci will be accomplished by using air displacements.

Fig. 1: Central nervous system of a cockroach
Keep these things in mind as you follow the
protocol below:
1) Be very conservative in the amount of CPS you apply to the animal: if you get the cerci wet it will be very hard to get good responses to wind puffs.
2) The crucial part of this experiment is the dissection. It is hard to
recognize the nerve cord if you have not seen it before. The TAs will prepare
one cockroach to the stage where the longitudinal muscle straps need to
be cut so that you can learn how to recognize the nerve. Dissect methodically,
using the microscope, taking the most care when you get near where the nerve
cord ought to be found. Do not mistake the tracheae (silvery) for the nerve
cord (almost transparent).
3) Lack of response from the nerve cord is often attributable to the electrodes
shorting out from touching each other or being immersed in a conducting
fluid such as CPS. Verify that the mineral oil-Vaseline mixture is properly
covering the electrode-nerve and that you haven't shorted the ends of the
hook electrode together.
4) Stabilizing the holder (petri dish) with dental wax along the base of
the dish will help eliminate accidental movement of the apparatus (usually
resulting in nerve injury).
5) Depending upon the stability of the preparation, you may need to push
air over the cerci in a direction away from the open nerve recording. This
can help eliminate undue movement of the recording leads and subsequent
baseline shifts.
Dissection
Select a large Periplaneta americana and
quickly cut off its head with scissors. Remove the insect's legs and wings
and pin the body ventral side down on the dissecting dish, making sure that
the pins are angled away from the body to reduce interference. After the
insect is pinned down, observe the composition of the two anal cerci under
the dissecting microscope.
Begin the dissection by making a longitudinal incision along the dorsal
plates (tergites) which cover the abdomen taking care not to damage the
tissue underneath. Once the dissection as begun make sure to keep the preparation
moist with cockroach perfusion fluid (CPS, Table 1).
Chemical |
Concentration |
| NaCl | 215.0 mM |
| KCl | 3.3 mM |
| NaHCO3 | 2.0 mM |
| CaCl2 | 1.8 mM |
Remove the tergites and push the gut and other organs to one side without rupturing them. Pick away the creamy white fatty tissue and tracheae, which appear as fine silvery tubes. The ventral cord is located beneath two strap like longitudinal muscles that will have to be carefully cut away. The cord itself is semitransparent and much thicker than the many surrounding tracheae and lies directly on top of the ventral cuticle or sternites. Take extreme care to when clearing away the tracheae, since they can be easily mistaken for the ventral cord and vice versa. Identify the terminal ganglia, which is the largest of the six abdominal ganglia (Figure 2) and locate the two cercal nerves protruding at the posterior ends at oblique angles. Carefully free a section of the nerve cord anterior to the terminal ganglia in order to slip the electrodes underneath.
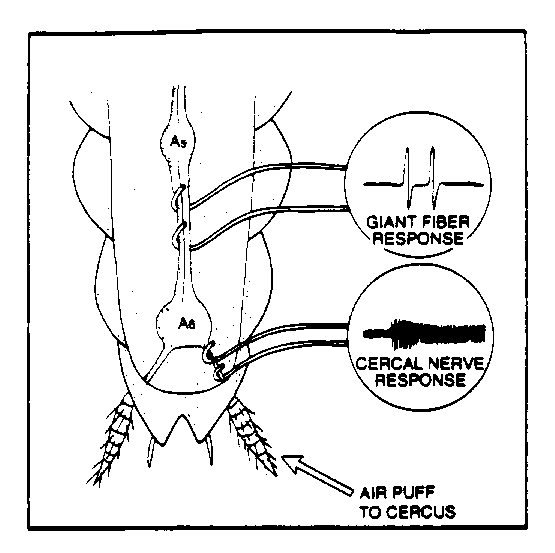
Fig. 2. Electrode placement for ventral and cercal (week 2) nerve recording.
Before recording the 3 silver wires (2 for recording and 1 for the ground)
must first be coated with silver-chloride to facilitate recording. This
will be done with the TAs. The ground electrode should be inserted into
the undissected portion of the thorax and connected to the common ground.
Carefully insert the recording electrodes under the ventral nerve cord and
lift it slightly. Using the twisted end of a Kimwipe, draw up any excess
CPS so that the fluid will not short out the recording electrodes. Use the
Vaseline-mineral oil mixture from a syringe to cover the hook electrode
and nerve to isolate from CPS that you will be reapplying to keep the preparation
moist. Connect the recording electrodes to the amplifier (WPI DAM60) input
and set the gain to 100-1000X, the low frequency filter 10 Hz, and the high
frequency filter to 3 kHz. Then connect the output of the amplifier to the
oscilloscope, audio amplifier, and MacLab. There should be some spontaneous
activity in the ventral nerve cord, adjust the settings on the amplifier
until this activity can be readily visualized on the oscilloscope and heard
on the audio amplifier. If a loud 'buzzing' noise is apparent (60 Hz noise),
then the leads and ground connections need to be adjusted. Once a consistent
signal is attained the experimental protocol can begin.
Experimental Protocol:
Air puff response. Make sure that the cerci are completely free of liquid and not touching anything (dish, pins, etc.). You may need to use a sipper/pippette to remove excess fluid. Using the supplied PE-205 tubing, blow on the cerci and observe the response. If there is no response, increase the intensity of the blast and/or stimulate the other cercus. Once you are satisfied that your prep is responding reasonably, stimulate only one of the cerci by placing a Kimwipe around the opposite cercus. Now use the flow meter and try to obtain a dose response using light, medium, and strong intensities of air displacement. Note down both the distance from cerci to end of PE tubing from flow meter and the LPM (liters per minute) you use. Try to use a fixed end-of-tube to cerci distance; you could try to use one of the micromanipulators for this purpose. Try this on both of the cerci and determine any differences in nerve cord response.
Ventral nerve conduction velocity. The velocity of an action potential in non-myelinated neurons is proportional to the diameter of the axon along which it travels. The ventral nerve cord of the cockroach contains several very large-diameter axons that originate in the last (6th) abdominal ganglion and extend up to the brain. It is easy to elicit action potentials in these so-called giant neurons (or giant fibers).
Calculation of conduction velocity is straightforward. Record an action potential at two different sites, and measure the time it takes for the potential to travel from the posterior electrodes to the anterior ones. Measure the physical distance between the electrodes. Finally, calculate the conduction velocity by dividing the distance by the time (expressing the result in meters/second).
Connect the hook electrodes from the posterior-most electrode into channel number 1 (CH1 on the MacLab/4 box), and the potentials from the anterior-most electrode into channel 2 (CH2). NOTE: check that the polarity of the two electrodes w.r.t. the neve is the same (i.e., if ground of one hook electrode is more posterior on the nerve, make sure that this is true of the other hook electrode)---do this by pulling on one of the two leads slightly. Blow on the PE tubing to stimulate the cerci and confirm you are recording the response of both hook electrodes; you will be using the 1K sampling mode so you may need to close down the filters and increase the gain in order for this to work.
Once you're recording setup is working, gently stimulate the cerci with the PE tubing while recording. From the records determine the latency between the posterior-most channel and the anterior-most channel. You will need to pick out some distinctive feature from the two records to correlate them. Measure the distance between the electrodes using the vernier micrometer; divide this distance by the measured latency and convert the units to obtain the velocity in meters/second. Do this 10 times and in your lab report present a histogram of the distribution of the conduction velocities.
Useful references for the conduction velocity portion of the protocol are:
Delcomyn, F. 1976. An approach to the study of neural activity during behaviour in insects. J. Insect Physiol. 22, 1223-1227.
Daley, D.L., Vardi, N., Appignani, B. & Camhi, J.M. 1981. Morphology
of the giant interneurons and cercal nerve projections of the American cockroach.
J. Comp. Neurol. 196, 41-52.
LABORATORY REPORT
Your lab report will cover both this weeks experiments and next week's:
due date is two weeks following next week's ventral nerve II lab.
Follow the standard journal format described in the syllabus. Some questions
you should address in your lab report are:
- What does a single spike on the screen actually represent?
- Are all the spikes the same amplitude? What does this mean?
- Do all the spikes have the same velocity?
- If you can see differences in spike amplitude, and if you can measure differences in spike velocity, is there any relationship between the two?
- Can you preferentially excite different spikes?
- How can you tell in which direction the spikes are traveling? Can you always be absolutely sure?
- How many neurons are there in the VNC (each connective). How many are you recording from?
- Why do you sometimes get a spike recorded at one electrode and apparently none recorded at the other?
- Why do insects have giant fibers?
WWW resource. A useful web page on the escape response of the American Cockroach.