Possibly the simplest electrophysiology experiment. Wire from speaker is used to stimulate a sensory hair or spine on the leg of a cockroach.
Lab #2
Recording from Cockroach Leg Mechanoreceptive Afferents
Equipment Needed Per Rig:
Standard. MacLab data acquisition system and Macintosh, MacLab Chart and Spike histogram extension software, 1 DAM60 Amp and connectors, Grass stimulator, oscilloscope and audio monitor, BNC cables, barrels, and Ts.(see Equipment page for model information etc. for lab report), male banana to BNC adapter
Special. 3 minuten pin electrodes, dental wax, 1 powered speaker with cover removed to glue stimulator wire onto, 9 cm solid stripped wire to glue to speaker, super glue.
Experimental Protocol:
1) Prepare a small platform with a dental wax bed to place roach leg on (once you have the leg, you should make a matching slight indentation in the wax to put the leg into for mechanical stability).
2) Superglue one end of an 8 cm piece of wire to the center of the powered speaker cone. Use a very small amount of superglue. It has a viscosity similar to water so you should either use a dabber or a pippette to apply the amounts needed (about one small drop per anchor point). You want the wire to be hanging straight down from this point when the speaker is lying on its side (see image below). Also, glue the point of contact with the plastic edge of the speaker (a lever is formed with the fulcrum at the speaker edge and driven by the cone).

Possibly the simplest electrophysiology experiment. Wire from speaker is used to stimulate a sensory hair or spine on the leg of a cockroach.
3) Setup dissection scope above platform -focus on the wax to make sure you have it setup properly. Setup surgery light. Connect signal path from output of amplifier on (audio monitor, scope, MacLab). Also connect stimulator to powered speaker, and to a second MacLab channel (NOTE: use lowest voltages available to start or you will blow out the speaker amplifier!!).
4) Anesthetize the cockroach with CO2 (done with TAs), and remove one leg at the proximal end of the coxa.
5) Examine leg under dissection scope. Identify as many anatomical and sensory features of the leg as you can distinguish, using Fig 1 of the Delcomyn et. al handout as a reference.
6) Connect electrodes to DAM60 and insert both ground electrodes and active electrode into leg as shown in the handout.

Positioning the wire and electrodes requires some care. The receptors are
directional; you need to match the plane of movement of the wire to the
particular hair you are recording from. Since it is difficult to stimulate
a single hair without also moving the leg, you may find after you've set
up the wire (a much more punctate stimulus, unlikely to move the entire
leg) that its movement does not cause any modulation of the firing rate:
you were fooled, and you need to reposition the wire onto a new hair or
reposition the electrode.
7) Once you've obtained signal that appears to contain the activity of mechanoreceptive afferents, you can begin probing the leg with a fine-tipped stimulator to see if the activity you are recording is driven by a particular tactile mechanoreceptor. Note that the less delicate your stimulation, the less likely it is that the resulting activity is from what you are stimulating (if you are causing mechanical deformation of the cuticle at some other portion of the leg, you could be observing the response of a campaniform sensilla located there).
8) Position your speaker and wire so that it is touching one hair or spine you have found to result in an increase in activity (you may need to move your electrodes if you haven't succeeded in doing this). Many of the tactile receptors are directional so you may need to fidget with the position of the wire and its plane of movement to obtain modulation of the afferent response.
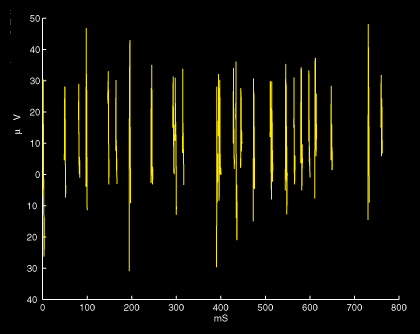
Graph of a response to a burst of stimulation from the speaker (spikes only).
9) Try to obtain the frequency response characteristics of at least one hair and spine. Record baseline activity; then you should try at least 3 frequencies so that you can try to graph the response as a function of frequency. Your amplitude should be fixed at some level that gives you a reasonable amount of response (but try to avoid saturation). Remember to record both the activity and the output of the stimulator. Note position of each hair or spine that you record from, and the effective plane of sensory organ movement. You can try to record in continuous mode (1K samples/sec) by closing the filters and increasing the gain as done during the EOD lab; but you will need confirm that this does not result in too much aliasing. If it does, use burst sampling mode.
10) Try to do an amplitude series on a single hair or spine. To do this, first confirm you can modulate the activity of the afferent with mechanical stimulation from the speaker. Now, reduce the amplitude to the point where you see no modulation of baseline rate. Increase in small steps and see if you can graph activity as a function of amplitude of stimulation (ideally this would be in terms of the amount of mechanical displacement; just use the value of the voltage going to the amplified speaker using the observed waveform on MacChart).
11) Try to determine if the putative target sensory organ drives phasic or tonic receptors. To do this, you won't be able to use the speaker since it will not provide a continual displacement: instead, try to do it by manually displacing the speaker wire. If there is sufficient time, you can instead mount a fine tipped stimulator in one of the micromanipulators and use it instead. Record the baseline activity: displace the sensory organ while recording the activity, and quantitate firing rate as a function of duration of the stimulus. The length of time you will need to record will depend on how phasic the receptors are.
12) Habituation. Using a single hair or spine, record baseline activity and then stimulate it with some reasonable frequency and amplitude for a duration of 30 seconds or so. Quantitate firing rate as a function of duration of stimulus.
13) Extra Credit (pick one):
A. How could the principles of mechanoreception be applied to aid in designing better cars (e.g. human-machine interface or car-road/environment interface etc)?
B. This lab crudely attempts to characterize one class of exteroceptors: tactile mechanoreceptors. Another important class of receptors in the cockroach leg serve as proprioceptors, a type of interoceptor. What is the relative importance of exteroception and interoception? Which do you think came first in evolution?
WWW resource. A useful web page on the neural basis of walking in the cockroach for writing the lab report.