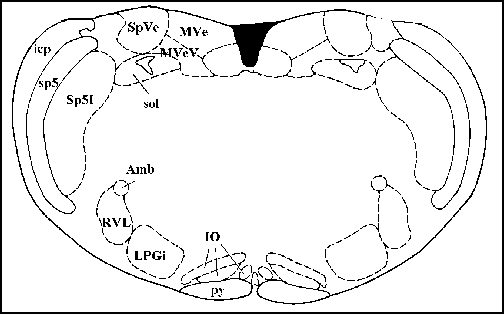
Cross section of the anatomy of the rat brain region you will be recording from
Lab #7
Autonomic responses in the VLM of the Rat
Equipment Needed:
Waldrop Lab Setup: Cardiovascular measurements are acquired with a Statham pressure transducer connected in series with a Gould transducer amplifier (arterial pressure) and a Gould Biotac amplifier (heart rate). Arterial pressure is monitored using a Kikousa 20 digital storage oscilloscope. Respiratory measurements are acquired by placing teflon-coated stainless steel wires into the diaphragm. These gross electrodes are connected to a high impedence probe (Gould) in series with a Gould P511 differential amplifier. This is connected to a Gould integrator amplifier (diaphragmatic EMG) in series with a Gould Biotac amplifier (respiratory frequency). All signals are sent to a Gould thermal paper chart recorder.
Stereotaxic apparatus include a Kopf headstage and bite bar. Attached to the headstage is a three-dimensional Kopf micromanipulator. Connected to this is a Frederick Hauer (FHC) motorized microdrive (1 micron resolution) and a WPI electrode holder.
Tungsten microelectrodes (2-10 M ohms) are used for extracellular recordings. Electrodes are connected to a high-impedence probe in series with a Gould P511 differential amplifier (100K gain, 300 Hz and 3 KHz filter stops). The output from the amplifier is sent to a FHC window discriminator. The window discriminator signal is sent to an FHC rate interval monitor, a Kikousa 20 digital storage oscilloscope, and a Tektronix 5115 analog oscilloscope. The output from the rate interval monitor is sent to Gould DC and Gould integrator amplifiers to record firing rate and TTL pulse activity, respectively. All recordings are made on a THC pneumatic vibration isolation table.
The temperature of the animal is constantly measured with a WSI rectal probe and monitor. Temperature is maintained between 36.5 and 38 degrees centigrade with a water-perfused heating pad and radient heat lamp.
Tissue cutting for histological analysis is performed with a freezing stage microtome (XXX).
Introduction
Neural control of the cardiovascular and respiratory systems is important for maintinence of homeostasis. The brain has a large impact upon functioning of these systems. One region of the mammalian brain, the medulla, has undergone significant study in regards to controlling cardiorespiratory functioning. Autonomic stimuli such as changes in blood pressure, hypoxia and exercise have been shown to change the activity of cells in this region of the brain. The ability of this region to alter cardiorespiratory functioning has been demonstrated with electrical and chemical stimulation and inhibition of this region. The goal of this lab, therefore, is to record neurons in the medulla, in particular the ventrolateral medulla, in response to various autonomic stimuli.
Dissection
Surgical preparation of the animal (done by the TAs): All surgical procedures meet the standards set by the NIH in Guidelines for Animal Experimentation. Rats will initially be anesthetized with an alpha-chloralose (65 mg/kg)/urethane (800 mg/kg) mixture i.p. and cannulated (external jugular and carotid with PE tubing) for further administration of drugs and measurement of arterial pressure (Statham transducer), respectively. Hindlimb withdrawal from noxious paw pinch and corneal reflexes will be used as tests for depth of anesthesia throughout the course of the experiment. Additional anesthetic will be administered intravenously as needed. A tracheotomy will be performed to allow administration of 100% oxygen during the course of the experiment; rats will be allowed to breath spontaneously. Rats will be placed in a stereotaxic head unit (Kopf) and temperature monitored via a rectal probe. Body temperature will be maintained between 36.5 and 38C with a water perfused heating pad as well as a radiant heat lamp. Electrodes will be placed into the diaphragm with a small gauge needle to measure diaphragmatic EMG and respiratory frequency. An occipital craniotomy will be performed to expose the dorsal surface of the cerebellum. The dura will be carefully excised with a small gauge needle to allow accurate, stererotaxic placement of a recording electrode through the cerebellum into the ventrolateral medulla. Fine, tungsten electrodes will be placed into brain at the appropriate stereotaxic level for extracellular recording of single neurons in the medulla. After experimentation, electrical lesions will be produced to mark the area in the brain. At the termination of the experiment, rats will be euthanised with an overdose of pentobarbital. The brain will be carefully removed and stored in formalin for histological analysis.
Histological analysis will include Nissl staining (Crestyl Violet) for
neuronal cell group identification and electrode placement reconstruction.
Brain slices will be cut in 50 micrometer sections and mounted and coverslipped
on subbed slides. The atlas of Paxinos and Watson (1986) will be used for
histological determination of recording sites.
Experimental Protocol:
Several different autonomic stimuli will be used in an attempt to record the responses of neurons which are responsible for controlling cardiorespiratory functioning. Increases in blood pressure will be induced by injecting phenylephrine (2-3 mg/kg) intravenously. Hypoxia gas (10% oxygen/90% nitrogen) will be administered to the animal for 30-60 seconds in order to induce systemic hypoxia.
Cardiorespiratory responses to these stimuli will be recorded with approximately 10 minutes between stimuli. The medullary neuronal responses to these stimuli will also be recorded.

Cross section of the anatomy of the rat brain region you will be recording
from
LABORATORY REPORT
Your lab report will be due on April 15, 1997 in standard format. Refer to Basic Lab Report Writing Tips for some new additional suggestions regarding methods
sections, along with some changes you'll welcome regarding inclusion of
filter settings and histogram extension settings in your lab report.
EXTRA CREDIT QUESTION
Autonomic functioning is a complex process involving various brain regions throught the level of the neuraxis. Based upon this idea, can you name any limitations regarding the findings of these experiments?