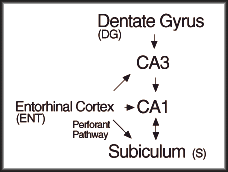
Place cell circuit tracks position.
Most animals must make willful, planned movement to succeed in life. A rat may have a den for pups and separate spots to scavenge for food. Caribou, arctic terns, and sperm whales travel great distances to follow seasonal blooms of food or to find a mate. The available sensory information and the distance to the target location determine the navigation strategy. When a human wakes in the dark, a mental or cognitive map of the room formed from experience can be combined with vestibular information and the length of a step to find the way to the door. Navigation based on idiothetic, internal, sensory information will accumulate errors (broken toes) the longer the path. Consistent landmarks help serve as markers on a larger scale. Locations defined by visual and/or olfactory cues with corresponding directions to the next landmark in the series defines a trail to the goal. Salmon locate exact spawning grounds aided by a unique blend of olfactory clues from the watershed for each stream. Animals reference reliable celestial landmarks like the sun, moon, and stars to navigate. Blackcap warblers migrate from Europe to subsaharan Africa using the stars. Homing pigeons use the magnetic field for navigation as well. All these sensory systems give the brain information to find the best path from one spot to another.
Animals must be able to answer life's navigation problems efficiently and flexibly. Arriving at the most efficient response to a challenge requires selection of relevant enviromental cues and the capability to plan an action based on memory of similar problems. Merriam's kangaroo rat can learn the distribution of food patches around its nest in three evenings of foraging. Marmoset monkeys reliably relocate food sites and do not revisit a place where food was already eaten on that foraging trip. Humans surgically treated for epilepsy by removing a small section of the right temporal lobe and the right hippocampus were deficient in similar spatial memory tasks where patients receiving the same treatment of the left hemisphere were not. Black-capped chickadees hide insects and seeds in numerous, widely spread caches in trees over its home range. The birds memorize visual cues to relocate hundreds of caches when winter creates a scarcity of food. Homing pigeons can use a sun compass and a sense of time to navigate. When the hippocampus, a part of the limbic system of the brain, is lesioned, homing pigeons can no longer learn the direction to a food source using the sun compass. Hippocampus-lesioned pigeons can still form memories. A pigeon with a lesioned hippocampus will learn a colored cue marks a location with food as well as a control pigeon with a normal hippocampus. The hippocampus lesion may impair spatial learning ability by preventing the coupling of direction information to location stimuli. How does the hippocampus function in spatial navigation?
Specific areas of the cortex and limbic system have primary roles in marking location and direction informed with sensory information from the modalities utilized by an animal. Visual cues for position have been used in most spatial experiments. The capability to associate tactile stimulus (shock) to a location has been demonstrated as well. A rat will explore and traverse a space forming a map of direction and position relative to prominent visual landmarks. The position and direction settings for a given area is stored in memory. The rat instantaneously recalls the spatial map when exposed to the area again. The brain must have awareness of current position and the direction in which the nose is pointing. Planned movement must be informed by an awareness of the starting point and the final location to achieve. The hippocampus and cortex select the straightest, most efficient path. Separate but interacting circuits of the limbic system and associated cortex monitor position or direction.
Place cells mark position. A place cell is defined by recording elevated rates of action potentials averaged over time in a particular location called the firing field. A particular cell will grade from a background rate of 1 action potential/second outside its firing field to an average maximum rate of 5-40 action potentials/second within the firing field. The function of average rate mapped over the area of the firing field appears as a steep peak. Firing fields do not have consistent areas, shapes, or density of overlap within a particular enviroment. The firing fields form within 2-3 minutes of exposure to a novel enviroment and are stable over months in identical conditions. However, a unique set of place cells may be active in the same enviroment in the dark. Place cells may take on combinatorial properties and fire only when a rat is walking a certain direction through the firing field on an arm of a radial maze for example. In an open arena, firing fields are omnidirectional, not subject to head direction (visual field). Furthermore, place cells do not fire in conjunction with a particular behavior performed within the firing field. Finally, the density of firing fields does not correlate with the amount of time spent in the area. As a rat walks within an enviroment, constellations of place cells respond with elevated average rates of action potentials as the rat passes through each place cell's firing field.

Place cell circuit tracks position.
The UCLA Laboratory of Neuro Imaging provides images of sections with the place circuit. Coronal Section (Select slice 10) Saggital Section (Select slice 9) Horizontal Section (Select slice 6)
Early classic:
O'Keefe J, Nadel L (1978) The hippocampus as a Cognitive Map. Oxford
University Press.
Reviews:
Eichenbaum, H (1996) Is the rodent hippocampus just for 'place'? Cur
Opin Neurobiol 6: 187-195.
Muller, R (1996) A quarter century of place cells. Neuron 17: 813-822.
Muller R, Ranck J, and Taube J (1996) Head dierection cells: properties and functional significance. Curr Opin Neurobiol 6: 196-206.
Some Recent Advances:
Ennaceur A, Neave N, and Aggleton JP (1996) Neurotoxic lesions of the perirhinal
cortex do not mimic the behavioural effects of fornix transection in the
rat. Behav Brain Res 80: 9-25.
Morris R, Pickering A, Abrahams S, Feigenbaum JD (1996) Space and the hippocampal formation in Humans. Brain Res Bull 40: 487-490.
Petersen K and Sherry DF (1996) No sex difference occurs in hippocampus, food-storing, or memory for foodcaches in black-capped chickadees. Behav Brain Res 79: 15-22.
