
A noctuid moth of the genus Catocala

Noctuid moths, like other nocturnally active flying insects, have exploited an ecological niche that is free of the predators (such as sharp-eyed birds) that are active during daylight. Unfortunately for moths, nighttime activity exposes them to another one of nature's most successful predators -- echolocating bats. Moth "ears," or tympanal organs, are located on the thorax and are sensitive to the ultrasonic frequencies used by bats, and therefore allow moths to hear the approaching bat. Since most noctuid moths do not use sound for their social interactions and since their tympanal organs are often most sensitive to frequencies of the echolocating calls used by the local bat population, it is thought that the auditory systems in most noctuid moths evolved as a direct result of bat predation (Fullard , 1987).
Tree of Life: Noctuoidea
Noctuid moths show a stereotyped two-phased behavior in response to the sounds of an echolocating bat. Weak ultrasonic stimulation (indicating a bat at a distance) generates negative phonotaxis, or a flying away response, to take the moth out of the detection range of the bat. Strong stimulation (indicating an approaching bat) produces either erratic behavior (e.g. unpredictable looping), or in a last ditch effort to escape, wing-folding to produce a free-fall. These responses occur at a short latency (40-100 ms) and field studies have been shown them to be often effective in avoiding bat predation (Roeder, 1967)
Noctuid moths have tympanal organs located bilaterally on the thorax. Each tympanal organ consists of only two receptor cells, A1 and A2. A third cell, cell B, is also located in the tympanal organ and is thought to subserve a proprioceptive role. A1 and A2 have very similar frequency response functions, but cell A1 is often about 10 times more sensitive than A2 (Roeder, 1967). It was initially speculated that these two cells mediate the two-phased response to acoustic stimulation. That is, activation of cell A1 by relatively low intensity sounds would initiate the negative phonotaxis response, whereas stimuli strong enough to excite both receptors would initiate the erratic flight/dive response. More recent work by Coro and Perez (1983) indicating inhibitory interactions between the receptors, and work by Waters (1996) demonstrating the importance of the temporal pattern of bat pulse emission in information processing by the moth, suggest that the mechanism is more complex.
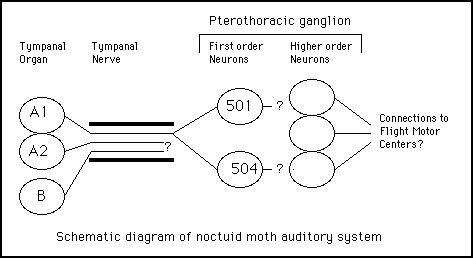
Centrally, cells A1, A2 and B project via the tympanal nerve to the pterothoracic ganglion. Boyan and Fullard (1986) have described the morphological and physiological properties of several identified auditory interneurons in this ganglion. Two of them (501 and 504) have been confirmed first order neurons, both receiving monosynaptic input from cell A1 (Boyan and Miller, 1991). Even though these two cells share an identical input, their different membrane properties allow these two cells to extract different features from the afferent input. Cell 501 has a higher response threshold and shows a nonsaturating I/O relationship while cell 504 shows a saturating I/O relationship. In addition, the decay constant for cell 504 is 3 times shorter than that of cell 501, allowing it to respond with greater fidelity to inputs of high repetition rates (as would occur during the terminal phase of bat hunting behavior). These differences would allow cell 504 to extract bat distance at close range, since it insensitive to changes in sound intensities above a certain level and it accurately encodes the high repetition rates used just prior to attack. These data support the idea that the temporal characteristics of bat calls are important parameters for determining bat distance.
Boyan and Fullard (1986) have also identified several higher order neurons in the pterothoracic ganglion. The role of these neurons in processing auditory information has yet to be clarified, but it has been shown that several of these neurons project to areas where flight motorneurons are found. It is possible that these connections may mediate the escape response by triggering motor programs for escape behavior.
There is a great deal of work yet to be done on the auditory system of noctuid moths. For example, how do moths discriminate bat from non-bat stimuli? In some tropical regions, there is continuous, intense sound produced by other chorusing insects that often has spectral components in the ultrasonic range. Since moths do not have an array of peripheral receptors (as vertebrates do) to analyze the spectral characteristics of sound, what temporal characteristics do they use to filter out this noise? This is an important problem to many auditory neurophysiologists since temporal cues are important to all animals (including humans) for sound pattern recognition. Another problem that moths must solve is localizing the direction in 3-D space of the hunting bat. How are the temporal and intensity cues from the two "ears" combined to produce an image of the location of the bat in space? Again, this is a problem that all animals with two ears have to solve. Noctuid moths provide an excellent model system to answer these questions since their peripheral auditory systems are extremely simple and remove the complication of spectral cues for sound pattern recognition. Furthermore, their auditory systems mediate a well characterized, stereotyped behavior that is important to their survival.
WWW resource:
Early classics:
Roeder K.D. and Treat A.E. (1957) Ultrasonic
reception by the tympanic organ of noctuid moths. J. Exp. Zool
134:127-157.
Roeder K.D. (1967) Nerve cells and insect behaviour. Cambridge: Harvard University Press.
Reviews:
Hoy R.R. (1992) The evolution of hearing in insects as an adaptation to
predation by bats. In: The evolutionary biology of hearing. Webster D.,
Fay R. and Popper A. eds. Springer-Verlag.
Fullard J.H. (1987) Sensory ecology and neuroethology of moths and bats: interactions in a global perspective. In: Recent advances in the study of bats. Fenton M., Racey P. and Rayner M.V. eds. Cambridge University Press.
Fenton M.B. and Fullard J.H. (1981) Moth hearing and the feeding strategies of bats. American Scientist 69: 266-275.
Some Recent Advances:
Coro F. and Perez M. (1983) Peripheral interaction in the tympanic organ
of a moth. Naturwissenschaften 70: 99-100.
Boyan G.S. and Fullard J.H. (1986) Interneurons responding to sound in the tobacco budworm moth Heliothis virescens (Noctuidae): morphological and physiological characteristics. J. Comp. Physiol. 158: 391-404.
Boyan G.S. and Miller L.A. (1991) Parallel processing of afferent input by identified interneurones in the auditory pathway of the noctuid moth Noctua pronuba J. Comp. Physiol. 168: 727-738.
Waters D.A. (1996) The peripheral auditory characteristics of noctuid moths: information encoding and endogenous noise. J. Exp. Biol. 199: 857-868.
