
A Sea Lamprey (Petromyzon marinus) affixed to a host

A Sea Lamprey (Petromyzon marinus) affixed to a host
North Americans are most familiar with the Sea Lamprey as the uninvited parasite that almost eliminated all of the native fish of the Upper Great Lakes after their introduction via the Welland Canal in 1921. Among many admirers, they have since overcome this notoriety (and their distinct lack of any redeeming aesthetic qualities) thanks to the efforts of Sten Grillner of Sweden and other neurobiologists over the past two decades who have made it a highly important model system for understanding vertebrate locomotion.
Sea Lamprey can live in the ocean or in fresh water, and go up rivers to spawn. Adult Sea Lamprey can reach 120 cm (47 in); landlocked Great Lakes P. marinus grow to 64 cm (25 in). They weigh 900-1400 grams (2-3 lbs), and have two dorsal fins and no scales. They have a mouth of sharp teath with a sucking disk. The mouth flares out when they are attaching to their hosts by suction, and their teath then rasps a wound into the host that is kept open with an anticoagulant conveniently generated by a buccal gland. They survive on the body fluids of their host, often until the host dies. The larval form lives in mud for 5 years and then transforms into an adult (control of lamprey populations in the Great Lakes is through a lampricide that kills the larvae).
Lamprey come from one of the oldest extant groups of vertebrates, having diverged from other vertebrates around 500 million years ago prior to the branch that led to modern fish, and have remained somewhat "primative" in terms of a number of measures of vertebrate complexity. Neurobiologically, they represent a proto-vertebrate, with a nervous system comprised of standard vertebrate cell types and structures, but orders of magnitude fewer neurons than modern vertebrates. The lamprey nervous system is also quite robust, allowing the kinds of long-term partial animal experiments that invertebrate neurobiologists typically use, and normally are very difficult in the modern vertebrates.
Tree of Life: Hyperoartia
The simplicity and robustness of the Sea Lamprey nervous system, along with an anguilliform mode of locomotion (movement by snaking their body forward and backward through the water) uncomplicated by pectoral fins and other advances of modern fish locomotion, have made it an ideal model system for studying vertebrate locomotion. Lamprey swim by propagating a laterally directed wave along their eel-like body. The amplitude of this wave increases towards the tail. The faster the wave is propagated along the body, the faster the lamprey swims. Steering is achieved by bending the body toward the desired direction. One complete cycle of forward and backward swimming is shown in the figure below. The total duration of this cycle varies from 4 seconds to 100 milliseconds (0.25 to 10 Hz), with a wavelength slightly less than the length of the body. A QuickTime movie of their method of locomotion may be found on Joseph Ayer's WWW page on a lamprey-based undulatory robot.
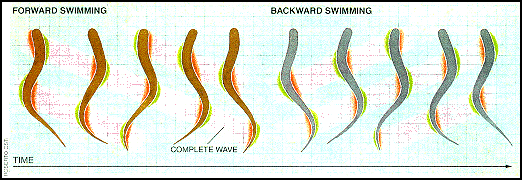
Rapid progress has been made in understanding the neural basis of lamprey locomotion over the last 15 years or so, with research proceeding on many levels---subcellular, cellular, segmental network, multisegmental network, computational, and behavioral.
The spinal cord consists of around 100 segments. Each segment contains around 1,000 neurons. They receive innervation from glutamatergic reticulospinal neurons in the posterior and middle rhombencephalic nuclei. This descending projection is what initiates locomotion, and may also provide tonic drive. They are in turn excited by rostral brain structures and by several different types of sensory stimuli, such as vestibular and lateral line (fish people will be fascinated to know that the lateral line of lamprey not only has neuromasts that sense water movement, but also photoreceptors on the skin of the tail region which are innervated by trunk lateral line nerves. Deliagina et. al has shown a flash of light on these sensors can initiate locomotion. Also very interesting is Northcutt's finding that the lamprey has ampullary electroreceptors). The laterally directed wave that is propagated along the body is due to alternating activation of motoneurons on the left and right side of each segment, in combination with successive activation of posterior (for forward swimmng) or anterior (for backward swimming) segments with a phase delay.
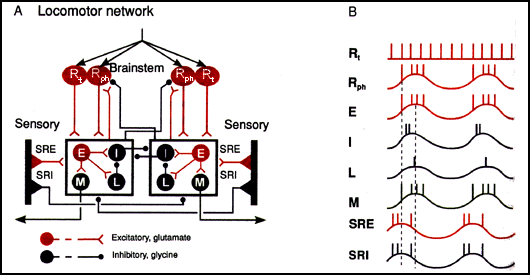
Circuit layout of one of the approx. 100 segments mediating lamprey swimming
along with brain stem innervation. Synapses terminating or originating on
box frames indicate an effect common to all neurons in the box. Filled circles
represent populations of neurons rather than individual units. Vertical
black bars are stretch receptors located on the lateral margin of the spinal
cord.
Abbreviations: Rt: tonically (continually) active reticular brainstem neurons Rph: phasically (intermittantly) active reticular brainstem neurons E: excitatory segmental interneurons I: inhibitory segmental interneurons L: lateral inhibitory interneurons M: motoneurons SRE: excitatory stretch-receptor neurons SRI: inhibitory stretch-receptor neurons
Circuit summary:
(A) Reticulospinal glutamatergic brainstem neurons Rt and Rph become excited and activate the first segmental neuronal network in the lamprey's spinal cord, exciting the E, I, L, and M groups of neurons on one side (hereafter referred to as the "active side") of the network. E neurons excite all the neurons in the box: the glycinergic inhibitory interneurons I that cross the midline and inhibit all the neurons in the other side of the network, the lateral interneurons L which inhibits the I interneuron, and the cholinergic M motoneurons that contract the swimming muscles. With a delay of 1% (since it is 1 of the 100 segments that comprise the length of the cord) of the total cycle time, the next segment in the direction of wave propagation is excited.
As the fish bends, SRE and SRI stretch receptor neurons on the active segment side are deactivated, while those on the contralateral side are excited by the extension caused by the contraction of the muscles on the active side. Thus, the SRIs of the inactive side begin to inhibit all the neurons in the box on the contralateral active side, and the SREs of the inactive side begin to excite all the neurons in the box on the inactive side.
The initiation of activity on the side opposite the currently active side is primarily a result of disinhibition of the neurons on the inactive side from the SRI neurons of the active side, in combination with a background excitatory drive.
(B) Diagram shematically showing the firing relationships of the key neuron groups on one side of a segmental neural network.
A Finer Resolution View
To understand some of the complicated dynamics of a single segment's network, imagine exciting one side of the network with an NMDA receptor agonist (which will initiate swim cycles of 0.1-3 Hz), or an AMPA/kainate receptor agonist (initiates swim cycles of 1-8 Hz). This will cause that half to be active, with the motoneurons contracting the myotomes they innervate on that side; the other side will be inhibited from activation by the active side's glycinergic inhibitory interneurons I that cross the midline. This is despite background excitation from Rt neurons. If nothing changes the lamprey will have continual asymmetric activity. How the network hops out of this seizure-like state is a useful way of approaching the system.
One way is sensory, already described above: extension of the inactive side causes SRI neurons to inhibit the active side, and the SRE neurons to activate the inactive side, which in combination with background excitatory drive becomes active.
Another way (that in reality acts in concert with the sensory burst termination mechanism) is through the electrochemical dynamics of the neurons on each side. The common terminus of these mechanisms is the manipulation of the calcium activated potassium channel current (IKca). When this outgoing current is turned on, the cell is repolarized and will stop firing action potentials.
One mechanism I'll describe is burst termination through ending what's called an NMDA plateau potential. As mentioned before, activation of a segmental network with an NMDA agonist will cause burst frequencies that correspond to slow swimming. In cells with NMDA receptors, sufficient excitation leads to activation of the voltage dependent NMDA channels, which leads to long lasting depolarization (the plateau potential). In an E cell this would result in continued excitation of the active side. But since the NMDA channel permits calcium to enter, calcium builds up in the cell. Eventually this turns on IKca and the plateau potential is ended. IKca inactivates slowly, leading to a long-lasting afterhyperpolarization (sAHP) in the cell. This not only terminates the activity on one side, it also results in spike frequency adaptation, since the sAHPs can accumulate with succesive action potentials, resulting in longer periods of nonfiring despite continued input. Post-inhibitory rebound of the inactive side is often achieved by the combination of background AMPA/kainate or NMDA activation in combination with the switching on of the low-voltage activated calcium current (LVA), that is only turned on just as the neuron is approaching action potential threshold from a previously inhibited state. Although this usually acts to give the inactive side an extra boost to start firing after disinhibition from the contralateral side, it can also contribute to IKca and subsequent repolarization.
Modulation of these dynamics is achieved through 5-HT (serotonin), GABAb, mGluR, and peptidergic systems. For example, 5-HT and dopamine are co-stored in a midline system of spinal neurons. This acts on the dendrites of the segmental network. 5-HT reduces IKca conductance, affecting the sAHP and NMDA plateau potential. It will cause longer bursts, and affects intersegmental phase delay. Dopamine reduces calcium currents, and so also contributes to IKca dynmics. Additional mechanisms of presynaptic modulation gate various neurons in the segmental networks so they are insenstive to supraspinal input during certain phases of the cycle of activation/inactivation, giving a measure of local control.
Currently, the wet-ware dynamics of single segments is better known empirically than multi-segmental coordination. It is known that during regular forward swimming there is a constant 1% phase delay between contiguous segments in a rostral-caudal direction. Isolated spinal cord shows the same pattern when activated by glutamate receptor agonists. This lag is variable however, and can be varied between +2% for forward swimming and -1% for backward swimming. Modeling in computo studies have shown that these phase delays can be accounted for given the currently understood pattern of segmental and intersegmental connectivity using simple neural mechanisms, but there is no consensus as to which model if any corresponds to in vivo reality.
In general, as is the case with stomatogastric ganglion dynamics, words quickly falter in giving an understanding of what's going on. As Grillner et al. stated in their '95 TINS review, in motivating the modeling approach, "Unfortunately, our brains cannot deal with this type of dynamic process, in which a number of factors are playing a role concurrently." Some very exciting computational neuroscience is being done by Örjan Ekeberg et al., who have created an integrated 3D neuro-mechanical model of lamprey locomotion that combines biophysically realistic neurons with simulated myotomes and water, and result in life-like patterns of virtual lamprey movement (see Ekeberg et al. '95). One interesting question for integrative neurobiologists is whether such breakthroughs might also leave human mechanistic-style (though not holistic informal) understanding behind, just as computer generated proofs of theorems do (see Special Topic references at the end of this page to follow this thread).
Early classics:
Rovainen, C.M. (1974a) Synaptic interactions of identified nerve cells in
the spinal cord of the sea lamprey. J. Comparative Neurology: 154:189-206.
Note: Click on author link to see a Citation Analysis of this paper.
Rovainen, C.M. (1974b) Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J. Comparative Neurology: 154:207-223.
Grillner, S., McClellan, A., and Perret, C. (1981) Entrainment of the spinal pattern generators for swimming by mechanosensitive elements in the lamprey spinal cord in vitro. Brain Research 217: 380-386.
Grillner, S. (1985) Neurobiological bases of rhythmic motor acts in vertebrates. Science 228: 143-49.
Reviews:
Nieuwenhuys, R. (1977) The lamprey brain in comparative perspective. Annals
of the New York Academy of Sciences 299:97-145.
Grillner, S. (1996) Neural networks for vertebrate locomotion. Scientific American, 274: 64-69. The full text of the article with figures is available on the Cure Paralysis Now WWW site.
Grillner, S., Deliagina, T, Ekeberg, Ö, Manira, A. El, Hill, R.H., Lansner, A., Orlovsky, G.N., and P. Wallén (1995) Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends in Neuroscience 18(6): 270-279.
Grillner, S., Wallén, P., Brodin, L. and A. Lansner (1991). Neuronal network generating locomotor behavior in lamprey: Circuitry, transmitters, membrane properties, and simulation. Annual Review of Neuroscience 14:169-199.
Some Recent Advances:
Buchanan, JT, and DR. McPherson (1995). The neuronal network for locomotion
in the lamprey spinal cord - evidence for the involvement of commissural
interneurons. Journal of Physiology (Paris) 89(4-6): 221-233.
Ekeberg, Ö., Lansnser, A. and S. Grillner (1995). The neural control of fish swimming studied through numerical simulations. Adaptive Behavior 3:363-384. Discusses the integrated neuro-mechanical model mentioned above.
(For another interesting integrated computational model of fish swimming and behavior see: Terzopoulos D., Tu X, Grzeszczuk R. (1995) Artificial fishes: autonomous locomotion, perception, behavior, and learning in a simulated physical world. Artificial Life: 1:327-351.)
Jung, R., Kiemel, T., and AH Cohen (1996). Dynamic behavior of a neural network model of locomotor control in the lamprey. Journal of Neurophysiology 75(3):1074-1086.
Schotland, JL, Shupliakov, O, Grillner, S, and L. Brodin (1996). Synaptic and nonsynaptic monoaminergic neuron systems in the lamprey spinal cord. Journal of Comparative Neurology 372(2): 229-244.
Vinay, L., Barthe, JY, and S. Grillner (1996). Central modulation of stretch receptors during fictive locomotion in lamprey. Journal of Neurophysiology 72(2):1224-1235.
Special Topic: Complexity versus Reductionistic Understanding
Anderson, P.W. (1972) More is different. Science 177:393-396.
Bullock, T.H. (1983). Epilogue: Neurobiological roots and neuroethological sprouts. In: Neuroethology and Behavioral Physiology, Huber F. and H. Markl eds. Springer-Verlag 1983.
De Millo, R., Lipton RJ, and AJ Perlis (1979). Social processes and proofs of theorems and programs. Communications of the ACM 22: 271-280.
Garfinkel, D. (1980). Computer modeling, complex biological systems, and their simplifications. American Journal of Physiology 239 (Regulative Integrative Comparative Physiology 8): R1-R6.
Selverston, A.I. (1980). Are central pattern generators understandable? The Behavioral and Brain Sciences 3, 535-571.
Weinberg, GM (1975). An Introduction to General Systems Thinking. New York: Wiley.
Full text of S. Grillner's Scientific American "Neural Networks for Control of Locomotion"
Experiments with a neuro-mechanical model of the lamprey
A Lamprey-Based Undulatory Robot
Great Lakes Lamprey Control Program
Fish Locomotion Bibliography and Abstracts
