American cockroachs, Periplaneta americana.

American cockroachs, Periplaneta americana.
A cockroach can streak at 70-80 cm/s while rapidly changing direction to escape a predator or a well-aimed shoe (Delcomyn 1985). The escape response is initiated by esquisitively sensitive hairs responding to the movement of sudden air currents around the last segment of the body called the abdomen. In the photograph above, long antennae are the primary tactile and chemosensory organs coming off the head. The wings and legs are attached to the thorax, the medial body segment. Finally arising from the end of the abdomen, the pair of anal cerci are covered with wind-sensitive hairs. An object rapidly approaching the cockroach pushes before it an air wave that alerts the cockroach to run for its life.
Tree of Life: Blattaria
A cockroach will respond to a tactile or wind stimulus directed at the body by rapidly turning away from the stimulus and running. The escape response due to air currents on the anal cerci is well studied. The sensory cells, central nervous system component, and motor response during running has also been investigated. The sensation and response of the cockroach up to the time where running begins is the current focus. A roach will begin running between 8.2 to 70.2 ms after a puff of air is directed at the anal cerci (Roeder, 1948). Typically, the first response is to turn. Sensory hairs respond to air coming from a particular direction. Turning involves a specific shift in the positions of the legs informed by the sensory hairs to prepare to launch. Both tactile pokes and air currents from the same direction elicit the same specific type of turn which suggests a common motor pathway after the sensory response (Schaefer et al., 1994). After the course is set, running starts.
Circuit summary:
The nervous system promotes survival of the cockroach by quickly activating
the running response in motor centers of the three thoracic ganglia. The
sensory nerves in the hairs send axons which terminate on the giant interneurons
(GI's) in the terminal abdominal ganglion (Dagan and Parnas 1970). Two equal
sets of 7 large diameter GI's have the capacity to quickly relay the sensory
information anterior along the ventral nerve cord through 5 intermediary
abdominal ganglia to the thoracic ganglia (Westin et al., 1977). Intercellular
stimulation of one of the 4 bilateral ventral GI's demonstrated that a running
cockroach turned away from the GI' s conducting a relatively greater proportion
of action potentials (Liebanthal et al., 1994). These results indicate that
the GI 's direct the appropriate escape direction. Two types of escape behavior
are elicited depending upon which GI' s are excited. The ventral GI' s mediate
running, and the dorsal mediate flying and running (Fouad et al., 1996;
Ritzmann et al., 1982). Tactile sensory neurons in the antennae and distributed
over the surface of the body lead to pathways that consolidate at the thoracic
locomotor centers (Fouad et al., 1996). As the turning behavior suggests,
different sensory signals converge on the same motoneurons. The type A thoracic
interneurons receive action potentials from the ventral giant interneurons
and direct excitatory or inhibitory input on the motoneurons or interneurons
controlling leg muscles (Schaefer et al., 1994). The supraesophageal and
subesopahgeal ganglia provide over-all modulation of motor activity. The
subesophageal ganglion is known to function in initiating motor programs
in insects. The parasitic wasp, Ampulex compressa , meticulously stings
a roach on the thorax and above the subesophageal ganglion before leading
it to a death chamber where the wasp's offspring will consume it alive.
The venom above the subesophageal ganglion eliminates the escape response
sealing the cockroaches doom. (Fouad et al., 1996).
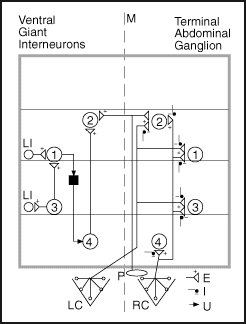
A diagram of the abdominal circuitry
Abbreviations: 1-4: Ventral giant neurons P: Peripheral motion sensor RC: Right Cercus LC: Left Cercus E: Excitatory synapse I: Inhibitory synapse U: Synapse type unknown M: Abdomen midline axis
Early classics:
Dagan D and Parnas I (1970) Giant Fibre and small fibre pathways involved
in the evasive response of the American cockroach, Periplaneta americana.
J exp Biol 52: 313-324.
Roeder KD (1948) Organization of the ascending giant fibre system in the cockroach, Periplaneta americana. J exp Biol 48: 545-567.
Ritzmann RE, Pollack AJ, Tobias, ML (1982) Flight activity mediated by intracellular stimulation of dorsal giant interneurones of the cockroach Periplaneta americana. J Comp Physiol 147: 313-322.
Westin J, Langberg JJ, and Camhi JM (1977) Responses of giant interneurons of the cockroach Periplaneta americana to wind puffs of different directions and velocities. J Comp Physiol 121: 307-324.
Some Recent Advances:
Fouad K, Liberstat F, and Rathmayer W (1996) Neuromodulation of the escape
behavior of the cockroach Periplaneta americana by the venom of
the parasitic wasp Ampulex compressa. J Comp Physiol A 178: 91-100.
Liebenthal E, Uhlmann O, and Camhi JM (1994) Critical parameters of the spike trains in a cell assembly: coding of turn direction by the giant interneurons of the cockroach. J Comp Physiol 174: 281-296.
Schaefer P, Kondagunta GV, Ritzman RE (1994) Motion analysis of escape movements evoked by tactile stimulation in the cockroach Periplaneta americana. J exp Biol 190: 287-294.
Cockroach WWW Resources:
